OUR PIPELINE
Developing next-generation polyene therapy

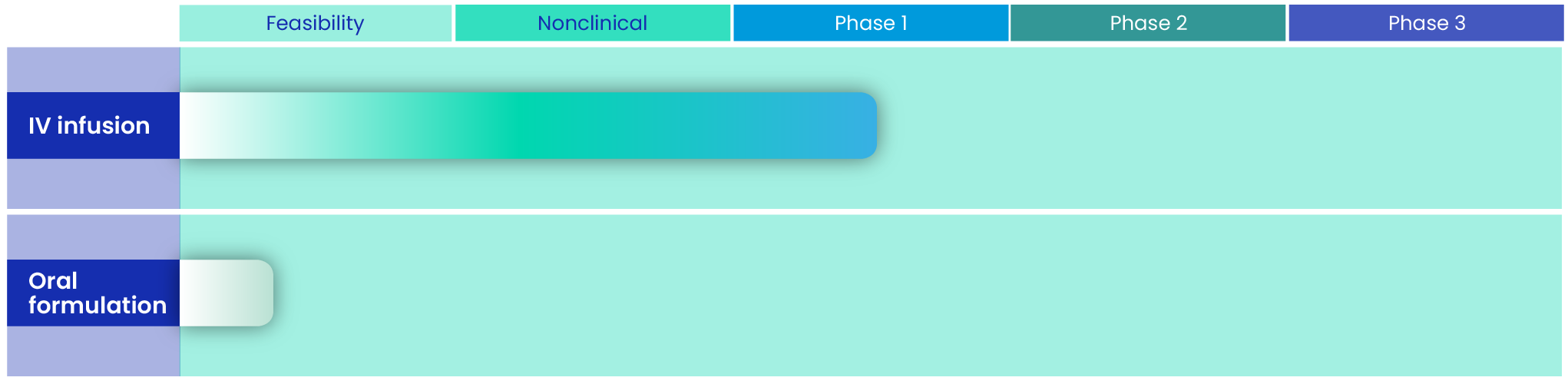
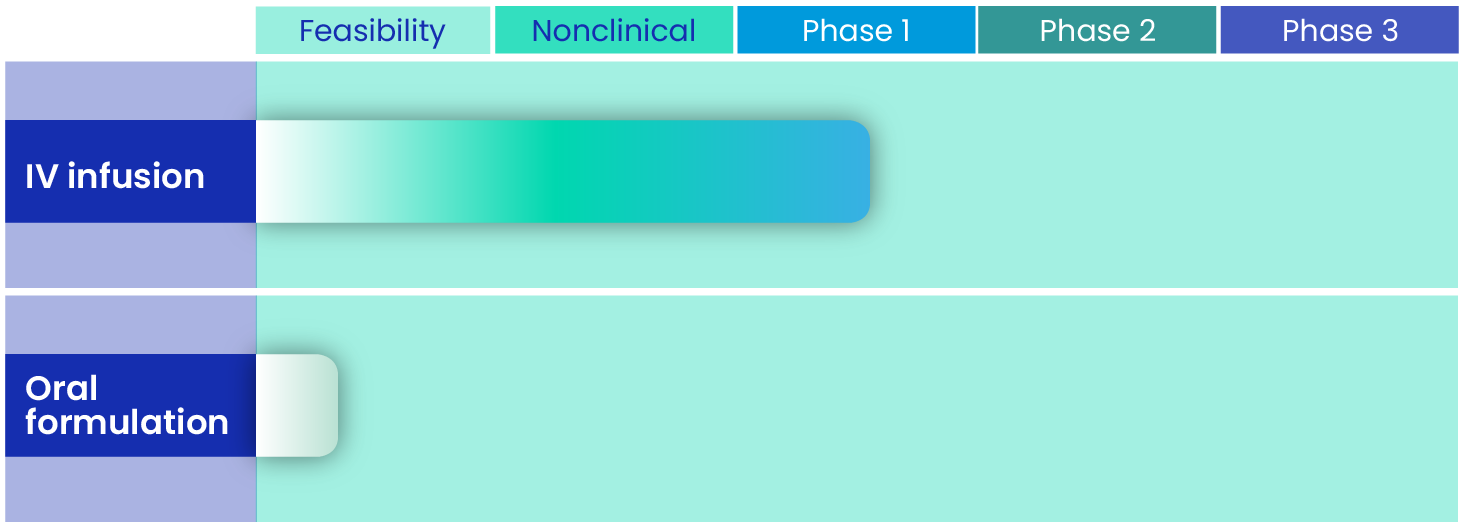
SF001 for IV infusion comprises a micellar solution of Sfu-AM2-19A, a novel, next-generation polyene. Nonclinical studies of SF001 have demonstrated its potent in vitro fungicidal activity and in vivo efficacy. The investigational new drug (IND) application was filed in May 2023, and clinical studies are underway.
An oral formulation is currently in the feasibility phase.
SF001 received Qualified Infectious Disease Product (QIDP) for invasive aspergillosis and Fast Track designations from the FDA in 2023.
Our SF001 clinical program
Qualified Infectious Disease Product Designation: An antifungal drug for human use intended to treat serious or life-threatening infections, including those caused by (1) an antibacterial or antifungal resistant pathogen, including novel or emerging infectious pathogens, or (2) qualifying pathogens listed by the Secretary under 505E(f) of the FD&C Act.
Fast Track Designation: An expedited program for drugs that are intended to treat serious or life-threatening conditions and have the potential to address unmet needs under section 506(b) of the FD&C Act.